Mini Project 1: Get Ur Gait On
Introduction
Our first Miniproject explores several topics in the biomechanical basis of terrestrial locomotion. In particular, we’ll focus on how well the inverted pendulum model applies in predicting the maximum speed of walking humans and to what extent some kinematic variables, like pelvic tilt, contribute to differences in speed. We’ll also look at how these kinematic variables vary by size and sex. Our general goals are to:
- Use imageJ to perform kinematic analysis
- Use simple mathematical models to predict maximum limb walking speed
- Test predictions of walking speed with measurements and observations
- Evaluate the effects of sex and size (scaling!) on your observations
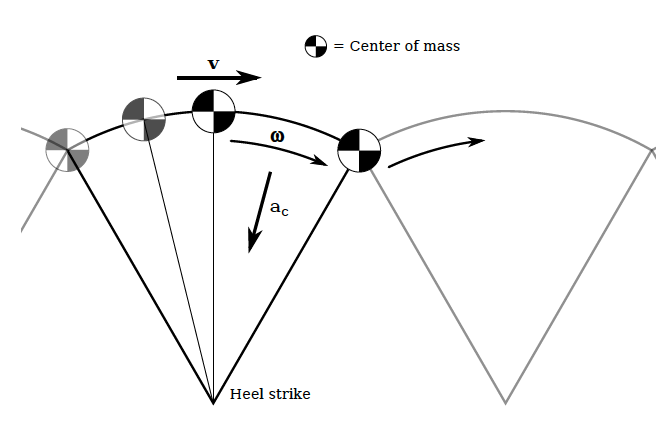
The inverted pendulum model of bipedal locomotion
Conceptual basis
As we’ve learned, the inverted pendulum (IP) is rather simple model for understanding how we walk. Under the IP model, the leg forms the radius of an arc through which your center of mass (COM) travels. Thus, we can use concepts surrounding centripetal forces to estimate how fast we walk. So far, we’ve ignored air resistance and the inertial effects of other moving parts (e.g., arms) and assumed the leg is rigid and rotates about the ground at a single point of contact. Under these conditions, we can make predictions as to the maximum linear velocity of our COM. The force associated with this swinging pendulum can be described as
\[ F =ma_c=\frac {mv^2}{R} \]
where \(a_c\) the acceleration due to gravity and \(R\) is the leg length, or the radius of the inverted pendulum. From this we see that the maximum velocity of the rotating mass is never greater than the square root of the product of gravity and the radius, or, in biomechanical terms
\[ v_{max}^2 \leq gl \]
\[ v_{max} \leq\sqrt{ gl} \]
where \(g\) is gravity and \(l\) the leg length. Thus, as we have learned in class, the Froude efficiency for walking is:
\[ Fr =\frac {v^2}{gL}<=1 \]
where \(v\) and any realized walking velocity is less than or equal to \(v_{max}\), or \(gL\).
As has become clear in our class discussions and demonstrations, the characteristic length of the pendulum (i.e., the radius) is the leg length and any increase in this length will result in a greater velocity. In addition, any biomechanical mechanism that increases the leg length during otherwise lower phases of the arc (e.g., lateral pelvic tilt, that is lifting the plant-leg side of the pelvis, ) will keep the COM higher and increase the velocity.
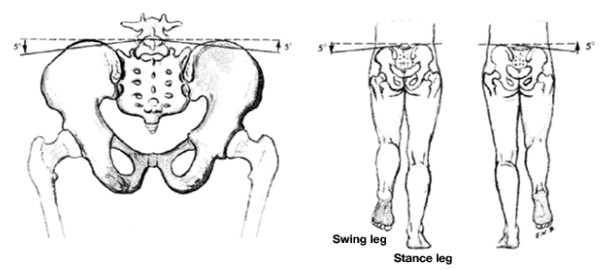
A walker tilting its pelvis
Methods
There are two parts to this MP: (1) predicting and testing predictions of maximum walking speed and (2) an analysis of pelvic tilt as it varies between normal walking at comfortable speeds and fast speeds. For both parts of this MP, it is important that you walk and not run. That means a duty factor of >0.5, i.e., you’ll always have a foot on the ground. Lab partners, police your colleagues to ensure this.
Sofware essentials
We’ll need a few pieces of software to analyze the kinematics of walking. First, download and install FIJI, a free image analysis package (FIJI is just imageJ) that just about everyone in the natural sciences uses at one point in their career. Second, enable the MTrackJ plugin by following these directions MTrackJ is a simple tracking routine that allows users to track points across video frames.
We’ll be working with video data captured with our phones. The file types associated with videos shot by phones are often unworkable in imageJ/FIJI. We need a way of converting your phone videos to AVI format, a file type that imageJ can open. Videos can be rendered into AVI files using—you guessed it—using R. See below
Max out your pendulum
For this part of the MP you will compare a predicted maximum walking velocity (\(v_{max}\)) to observed maximum walking speed. First things first: mark off a distance of 5 meters on level ground (e.g., in the hall, atrium, etc.).
With your knees locked and your hands by your side and not swinging, walk on your heels as fast as you can across the the marked distance. Concentrate on not using any movement in your pelvis or knees. This may require some practice. You may, if you’re careful, have a lab partner push you gently to get you going at the start of the 5-m field. With a phone or watch, record the time it takes to traverse the 5-m distance.
Using these data, compute the velocity (in m s\(^{-1}\)) and compare this to your calculated \(v_{max}\) based on theory. Please enter these data HERE. They can be accessed here for later analysis as well.
Full-tilt walking
The radius of your rotating mass can be effectively increased by subtle changes in posture, including active alterations in pelvic position. Lateral pelvic tilt is one such mechanism and the angle of tilt can be measured using ImageJ. While pelvic tilt results in a lengthening of the plant-leg, there are other postural consequences.
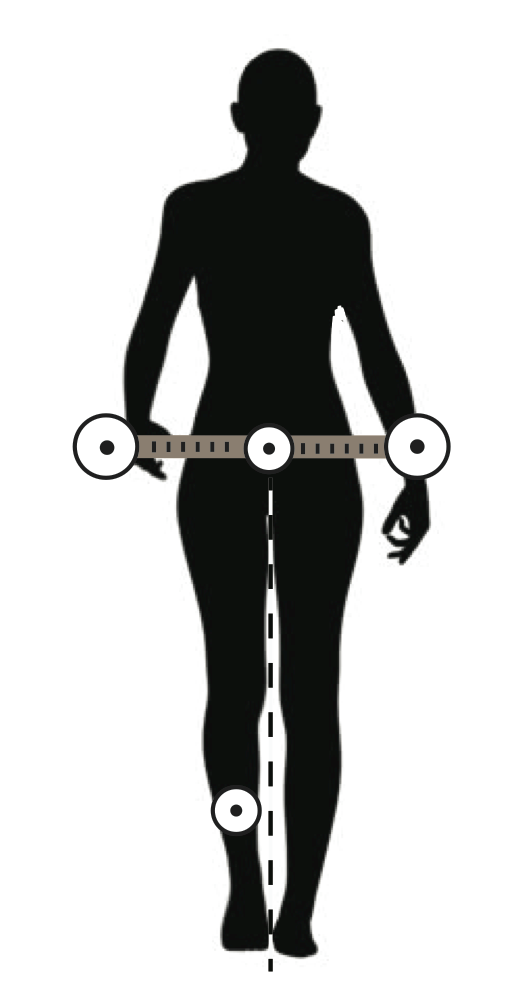
Start with donning the markers and the yardstick with markers attached at its end as in the figure below. Attach one small marker low on the front of your shin. Attach the yardstick with binder clips to your pantwaist. Be sure that the yard stick rests right above your anterior superior illiac spines (those bony points on the front of your hip). This apparatus will amplify your pelvic motions and make data recording much easier. Have a project partner record video (in normal video mode) of your walk as you walk toward them over the 5-m course from the earlier experiment. Be careful that the full width of the yardstick in the frame at all times. You’ll perform two trials per partner: (1) walking normally at a comfortable speed and (2) walking normally but as quickly as possible. For both trials, use ImageJ and the MTrackJ plugin to track the markers on your body. From these data, you should compute pelvic tilt angle (i.e. the difference between the maximum and minimum tilt angle). Please also enter these data here. Remember, these data can be accessed for analysis from this directory. Also notice there are data from previous iterations of the class. Be sure to use all of these in your analysis.
To analyze your videos in ImageJ, you’ll need to convert them to and AVI file and have the MtrackJ plugin installed (see below). Once the AVI file is stored in a directory, open it with ImageJ (File\(\rightarrow\) Import \(\rightarrow\) AVI . . .). Launch the MTrackJ plugin (Plugins \(\rightarrow\) MtrackJ) and follow the directions from the online manual. The goal here is to track four positions and calculate your two kinematic values: pelvic tilt and leg angle.
Converting Videos
We need a way of converting our phone videos into a format readable
by FIJI/imageJ. The AVI format is essentially a stack of raw images that
is readable by almost any software. So let’s convert our videos to AVI.
Fortunately, R can do that for us if we have the av package
installed.
library(av)
#set your working directory
setwd("some_directory")
#list files ending in MOV, the format stored by iphones
f <- list.files(full.names = T,pattern=".MOV")
#create a place to store images
dir.create("images")
#for each file, f, breakup the .MOV into the images and build avi files
for(i in f){
if(dir.exists("images")) unlink("images",recursive = T)
#extract images
av_video_images(i,destdir ="images",format="tiff")
#list images
f.i <- list.files("images",full.names = T)
#make the avi from the images from the MOV
av_encode_video(f.i,gsub("MOV","avi",basename(i)),codec = "rawvideo")
}
You lookin’ all marked and stuff
Metadata
After converting your videos, you’ll have to assemble a bunch of data
from them. Fortunately, we’ve learned how to do this lately. However,
for it to proceed smoothly, you might need some guidance, especially
dealing with the data from the MTrackJ analysis. Let’s assume you
produced 4 files for two teammates that each walked fast and slowly. If
we had our data file names begin with “walk”, we could use the handy
pattern argument in list.files() to find all
the files we want to analyze in the directory.
## [1] "walk_fast_me.txt" "walk_fast_you.txt" "walk_slow_me.txt"
## [4] "walk_slow_you.txt"Notice how they are similarly named “walk_speed_subject.txt”. That familiar convention of adding metadata to the filename makes it easy to extract “data about the data” from the file names. Now, as we did in the first AME1, let’s concatenate all the data into one data frame for subsequent plotting and analysis.
walk.dat <- list() #make a list for storing
for(i in walk.files){
walk.i <- read_delim(i,delim="\t") #read data as a tibble
met.dat <- unlist(strsplit(i,"_")) #split i for metadata and unlist it
speed <- met.dat[2] #get speed
subject <- gsub(".txt","",met.dat[3]) #remove ".txt"
walk.dat[[i]] <- walk.i%>%
mutate(speed=speed,subject=subject)
}## Rows: 150 Columns: 14
## ── Column specification ──────────────────────────────────
## Delimiter: "\t"
## dbl (12): Nr, TID, PID, x [pixel], y [pixel], t [sec], I [val], Len [pixel],...
## lgl (2): D2R [pixel], v [pixel/sec]
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
## Rows: 150 Columns: 14
## ── Column specification ──────────────────────────────────
## Delimiter: "\t"
## dbl (12): Nr, TID, PID, x [pixel], y [pixel], t [sec], I [val], Len [pixel],...
## lgl (2): D2R [pixel], v [pixel/sec]
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
## Rows: 150 Columns: 14
## ── Column specification ──────────────────────────────────
## Delimiter: "\t"
## dbl (12): Nr, TID, PID, x [pixel], y [pixel], t [sec], I [val], Len [pixel],...
## lgl (2): D2R [pixel], v [pixel/sec]
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.
## Rows: 150 Columns: 14
## ── Column specification ──────────────────────────────────
## Delimiter: "\t"
## dbl (12): Nr, TID, PID, x [pixel], y [pixel], t [sec], I [val], Len [pixel],...
## lgl (2): D2R [pixel], v [pixel/sec]
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.## # A tibble: 6 × 16
## Nr TID PID `x [pixel]` `y [pixel]` `t [sec]` `I [val]` `Len [pixel]`
## <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
## 1 1 1 1 200. 252. 0 255 0
## 2 2 1 2 200. 253. 0 255 0.31
## 3 3 1 3 200. 253. 0 251 0.589
## 4 4 1 4 200. 253. 0 246 0.91
## 5 5 1 5 199. 253. 0 241 1.17
## 6 6 1 6 199. 254. 0 255 1.42
## # ℹ 8 more variables: `D2S [pixel]` <dbl>, `D2R [pixel]` <lgl>,
## # `D2P [pixel]` <dbl>, `v [pixel/sec]` <lgl>, `? [deg]` <dbl>,
## # `?? [deg]` <dbl>, speed <chr>, subject <chr>Sweet. But, notice that the column names given to the data are
gobblygook with all those spaces in and “[pixel]” and “[sec]” in there.
Let’s change them for easy referencing with a str_remove()
(remove string) operation from thestringr package loaded
with tidyverse . . .
## # A tibble: 6 × 16
## Nr TID PID x y t `I [val]` Len D2S D2R D2P
## <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <lgl> <dbl>
## 1 1 1 1 200. 252. 0 255 0 0 NA NA
## 2 2 1 2 200. 253. 0 255 0.31 0.31 NA 0.31
## 3 3 1 3 200. 253. 0 251 0.589 0.589 NA 0.279
## 4 4 1 4 200. 253. 0 246 0.91 0.908 NA 0.321
## 5 5 1 5 199. 253. 0 241 1.17 1.16 NA 0.256
## 6 6 1 6 199. 254. 0 255 1.42 1.41 NA 0.249
## # ℹ 5 more variables: `v [pixel/sec]` <lgl>, `? [deg]` <dbl>, `?? [deg]` <dbl>,
## # speed <chr>, subject <chr>That’s better. Now, let’s pare down the data to those columns we actually care about, “x” and “y”, the x and y positions of the points, “subject”, and “speed”. Also notice that the MTrackJ file contains a “TID” and “PID” column identifying to what track (which dot in the image) and point (the series of positions) to which the x and y positions belong. So let’s keep these columns, too, then dump the others, and give it a quick plot with ggplot.
walk.dat2 <- walk.dat%>%
select(TID,PID,x,y,subject,speed)
walk.dat2%>%
ggplot(aes(x,y,col=speed))+geom_point()+facet_grid(subject~.)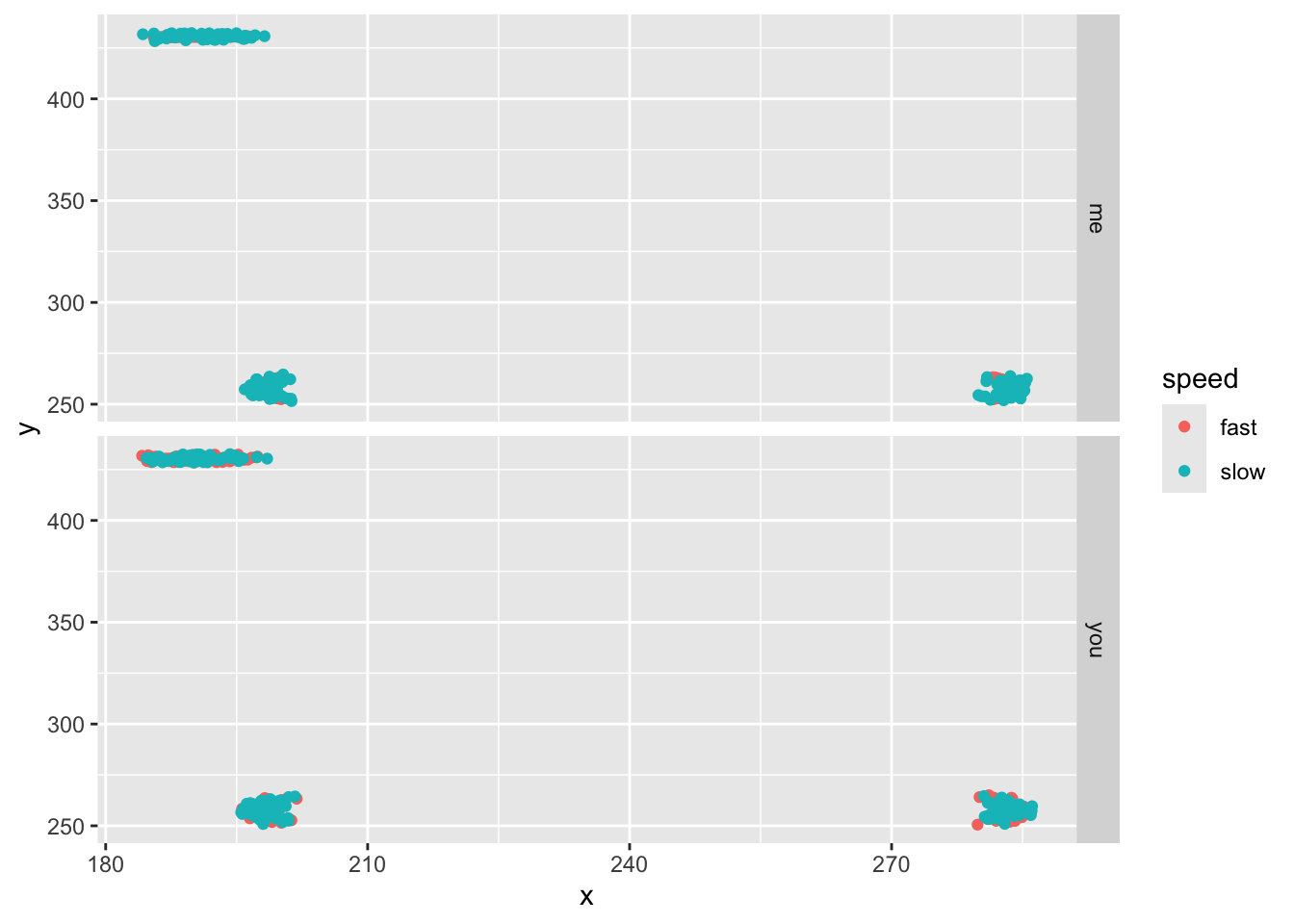
Notice that our leg point (TID=3) is higher than the hip. That’s odd! Well, not really. Convention holds that pixel 0,0 is the upper left of the image, so that means the coordinates are flipped vertically in our images. So let’s flip them back by finding the absolution value each y value minus the maximum y position (the image height).
walk.dat2 <- walk.dat2%>%
group_by(speed,subject)%>%
mutate(y=abs(y-max(y)))
walk.dat2%>%
ggplot(aes(x,y,col=speed))+geom_point()+facet_grid(subject~.)#grid it with columns = to speed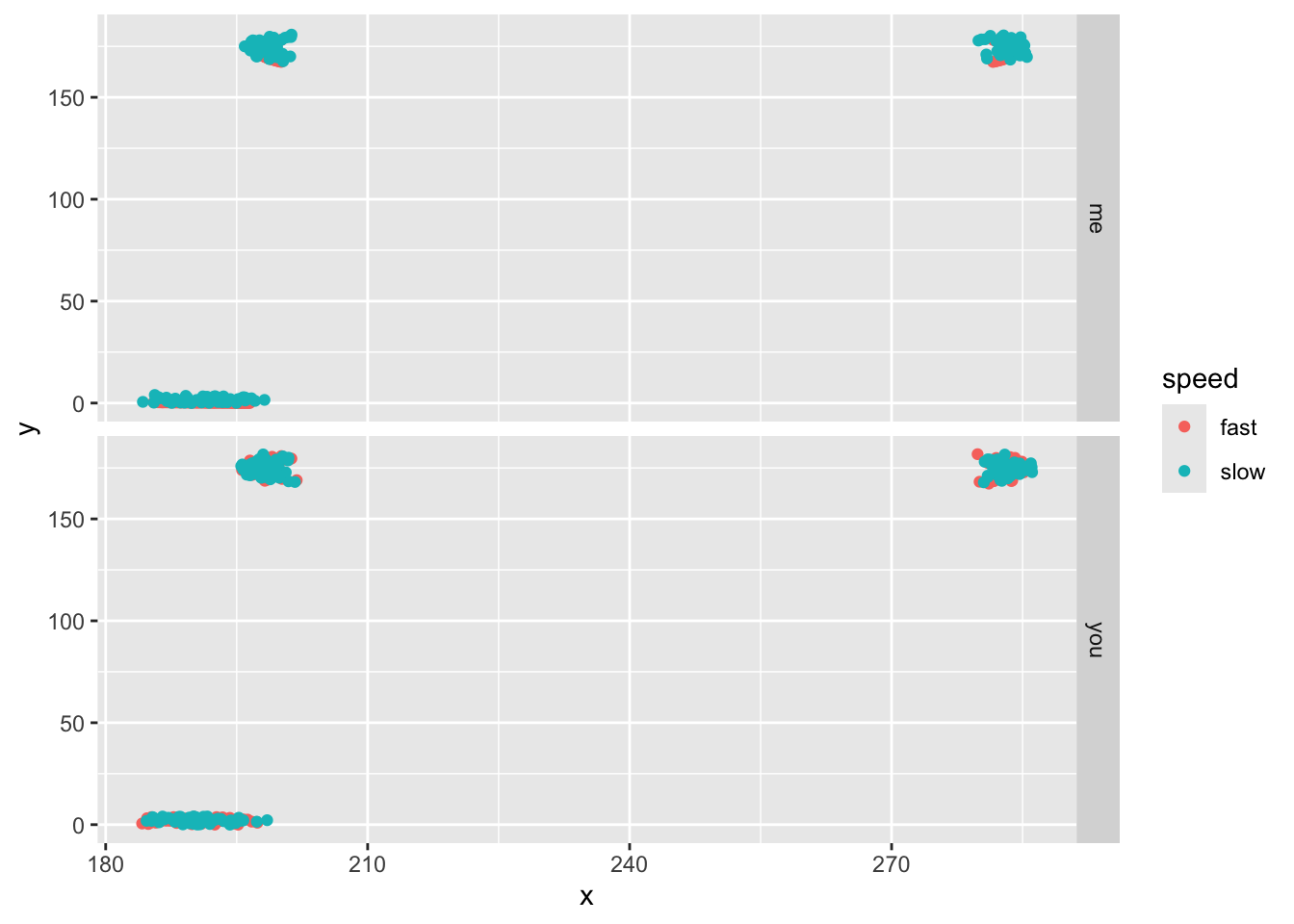
OK, all well ’n good. But does it make sense to identify our tracked points by “TID”, that is 1, 2, and 3? Wouldn’t it be better to change this to some character description like “hipR”, “hipC”, and “legR”, for right hip, center of the hip, and right leg, respectively. Let’s do it by changing TID to these strings using a series of mutate operations that replace each TID value. (if you do this, confirm the order is correct!)
walk.dat2 <- walk.dat2%>%
mutate(TID=replace(TID, TID==1, "hipR"))%>%
mutate(TID=replace(TID, TID==2, "hipC"))%>%
mutate(TID=replace(TID, TID==3, "legR"))
head(walk.dat2)## # A tibble: 6 × 6
## # Groups: speed, subject [1]
## TID PID x y subject speed
## <chr> <dbl> <dbl> <dbl> <chr> <chr>
## 1 hipR 1 200. 178. me fast
## 2 hipR 2 200. 178. me fast
## 3 hipR 3 200. 178. me fast
## 4 hipR 4 200. 178. me fast
## 5 hipR 5 199. 177. me fast
## 6 hipR 6 199. 177. me fastNow it’ll be easier to keep track of which track we intend to reference in any plotting or analysis.
Long vs. Wide data
With the data loaded, let’s think about what sort of operations you need to produce on this data set. You need angle for pelvic tilt, right? The angle subtended by the tip of our meter-stick amplifiers and the center of the pelvis (\(\theta\)) can be described in the following:
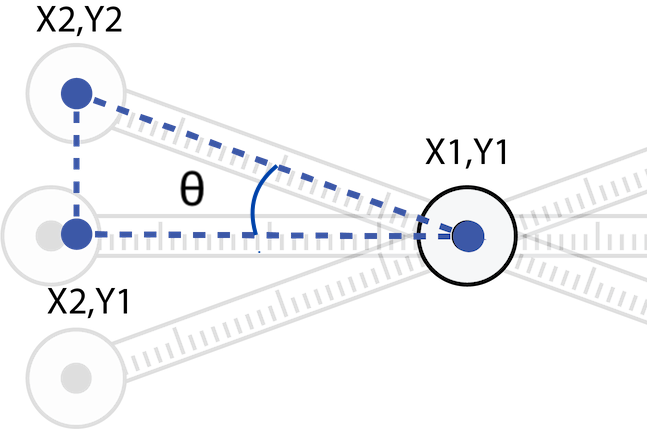
A look at the coordinates of the amplified pelvic positions
where points \(x_1y_1\), \(x_2y_2\), and \(x_2y_1\) respectively describe the coordinates of the pelvic center, the tip of the pelvic amplifier, and a theoretical point that represents a vertex horizontally aligned with the pelvis and vertically aligned with the hip amplifier. In other words, \(x_1y_1\) describes the coordinates of “hipC” and \(x_2y_2\) describes the coordinates of “hipR” in x and y pixels in the data above. So arranged, we have a right triangle with vertices described by the x and y pixels we have digitized with MTrackJ. With simple trigonometry, we can calculate \(\theta\). So please think about how to do this with calculations in R.
The goal here is to calculate \(\theta\) over every frame. But we have a problem. Notice that the data, as presented above, don’t have the x and y pixels for each point aligned in unique rows according to each frame (“PID”). That is, the data are in a “long” format where the column “TID” (the point name) describes “PID” (the frame). Put another way, x and y pixel data are repeated in succession down the table for each point name. This makes calculations for frame-specific \(\theta\), which will presumably require using the x and y coordinates for three points, very cumbersome.
Not to worry, however. Data wonks are accustomed to this problem and we can simply transform the table to a “wide” format. By wide, we mean that, for each frame, we’ll have sepqrate x and y columns for each point name, making frame-specific calculations based on these points easy peasy.
Here’s how it’s done:
## # A tibble: 6 × 9
## # Groups: speed, subject [1]
## PID subject speed x_hipR x_hipC x_legR y_hipR y_hipC y_legR
## <dbl> <chr> <chr> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
## 1 1 me fast 200. 282. 185. 178. 167. 0.381
## 2 2 me fast 200. 282. 186. 178. 168. 0.394
## 3 3 me fast 200. 282. 186. 178. 168. 0.392
## 4 4 me fast 200. 282. 186. 178. 168. 0.370
## 5 5 me fast 199. 282. 186. 177. 168. 0.331
## 6 6 me fast 199. 283. 187. 177. 168. 0.310Notice that we now have columns that are named as “x_” and “y_” with
a point name suffix, i.e., we have taken the x and y column and made new
ones based the point they describe. We have several new columns and
fewer rows of course. For this we used dplyr’s
pivot_wider() functions that, well, pivots long data to
wide data. We told pivot_wider() to take the names from
“TID” (the point names) to form the new columns with values from the
columns named “x” and “y”. We could now compute \(\theta\) for each PID (i.e., frame) based
on the coordinates of each point.
Adding references
As we take on the rest of the mini-projects, including this one, we’ll be reading scientific papers so that we can place our results in context. In addition, as scientists, we always want to support our statements of fact with references. For this, we’ll need to cite and include these papers in a bibliography.
Adding reference to an .Rmd is pretty straight forward. First we’ll need to establish a .bib file that will reside in the same directory as your .Rmd file. To do this, select “File->New File->Text File.” A new tab will appear with the name “Untitled1.txt”. Save this file as “BIOL5380.bib” (note the “.bib” extension) in the same directory as your .Rmd file. This .bib file will be referenced by your .Rmd when inserting citations.
The framework for citations and references in .Rmd is formulated around BibTex. BibTex is handy because you can find BibTex formatted citations in all your typical outlets (see note below). This system of citation uses a .bib file that contains text describing published works. Each work or potential reference in the .bib file is identified with a unique tag at the top of its entry and this entry contains the important bibliographic information. For instance, a .bib file containing a reference to the following paper
Usherwood, J.R., 2005. Why not walk faster?. Biology Letters, 1(3), pp.338-341.
would have the following text:
@article{usherwood2005not,
title={Why not walk faster?},
author={Usherwood, James Richard},
journal={Biology Letters},
volume={1},
number={3},
pages={338--341},
year={2005}
}Notice we have a tag “@article{usherwood2005not,”. This identifies the entry as a journal article (as apposed to a book or manual, etc.) and contains a key that is unique among all the other references in the .bib file. After this, the entry then goes on to identify the important bibliographic pieces: title, author, journal, etc.). Go ahead and add this BibTex reference to your BIOL5380.bib file and save it. For .Rmd to reference this .bib file, we need to specify a bibliography file in the YAML header. So now alter your YAML header at the top of the .Rmd so that it contains `bibliography: BIOL5380.bib”. The YAML header should now look something like this:
---
title: "Mini-Project 1"
author: The Inverted Pendula
date: 21/Jan/2024
output: html_document
bibliography: BIOL5380.bib
---With a .bib file now in place, we can use the reference keys to cite
works listed in the .bib file. For this, we simply add the key label to
the text with ‘@’. For instance, @usherwood2005not will
produce Usherwood (2005).
When citing published works in text, we often follow certain
formatting conventions. To be honest, your professor couldn’t care less
about which format gets used in your .Rmds. Fortunately, R Markdown uses
a familiar Harvard-like
format by default and he recommends plowing ahead with it. In a
Harvard-like format, a reference in a sentence used in an explicit
manner includes the reference year in parentheses. For instance, if we
were to express that these authors studied the the Froude number in
humans, we would write, “Underwood (2005) studied the Froude number in
human walking.” The reference usherwood2005not is just
inserted at the beginning of the sentence as in the following:
@usherwood2005not studied the Froude number in human walking.However, Harvard-like convention follows that if we aren’t explicit about the study, we include the entire reference in parentheses. For instance, if we wanted to write, “A recent study considered the Froude number in human walking. (Usherwood 2005).” we would include the following:
A recent study considered the Froude number in human walking. [@usherwood2005not].Note that usherwood2005not is wrapped in
[].
In R Markdown, bibliographies will be placed at the end of the document (see below). Normally, this means you want to end your document with the header “References”. This will anchor the bibliography in this section.
Note that this .bib file can be used in all your .Rmd documents, not just this one. As long as all the BibTex entries have unique keys, this .bib file can serve as a reference database for all your .Rmd citations.
You may be wondering where you can find BibTex citations. Like so much of our lives, the answer points to Google, specifically Google Scholar. As an example, go to Google Scholar and search for the Usherwood (2005) paper. The entry under scholar will look like the following:
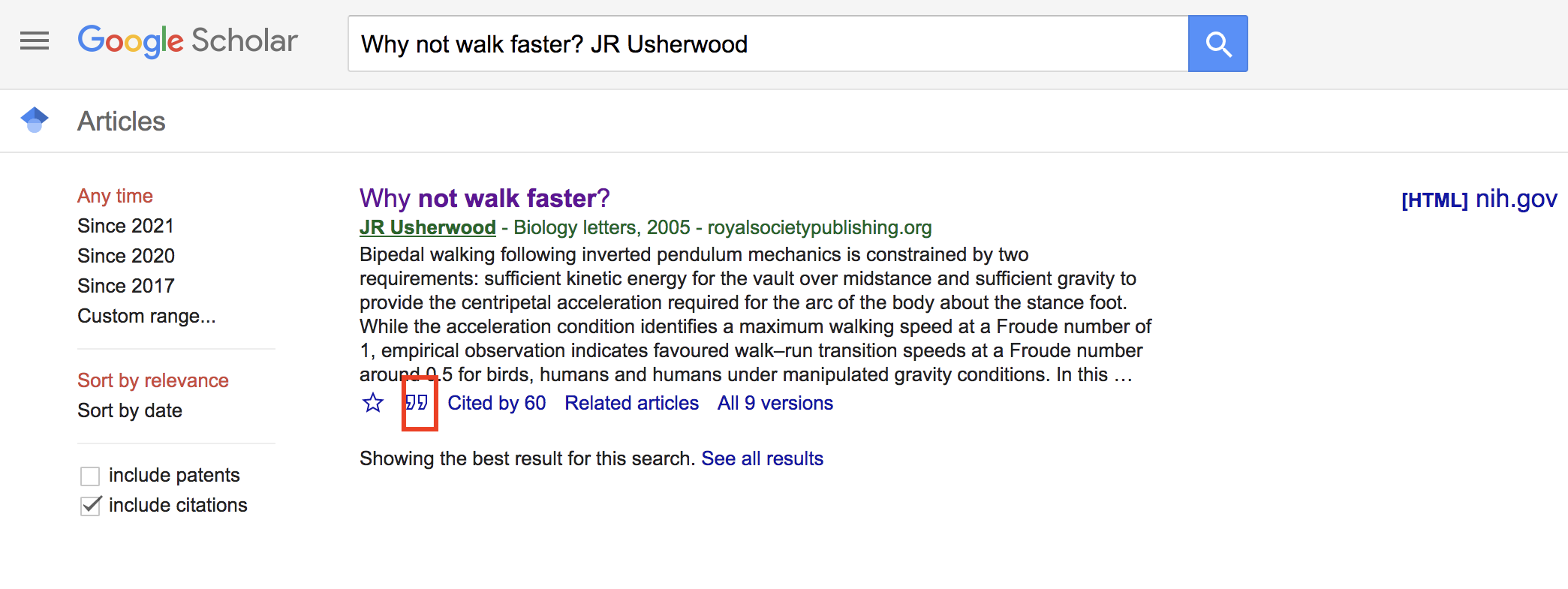
Click on the big " at the bottom of the entry. This will
bring you to a pop-up window that contains different citation formats
for the entry.
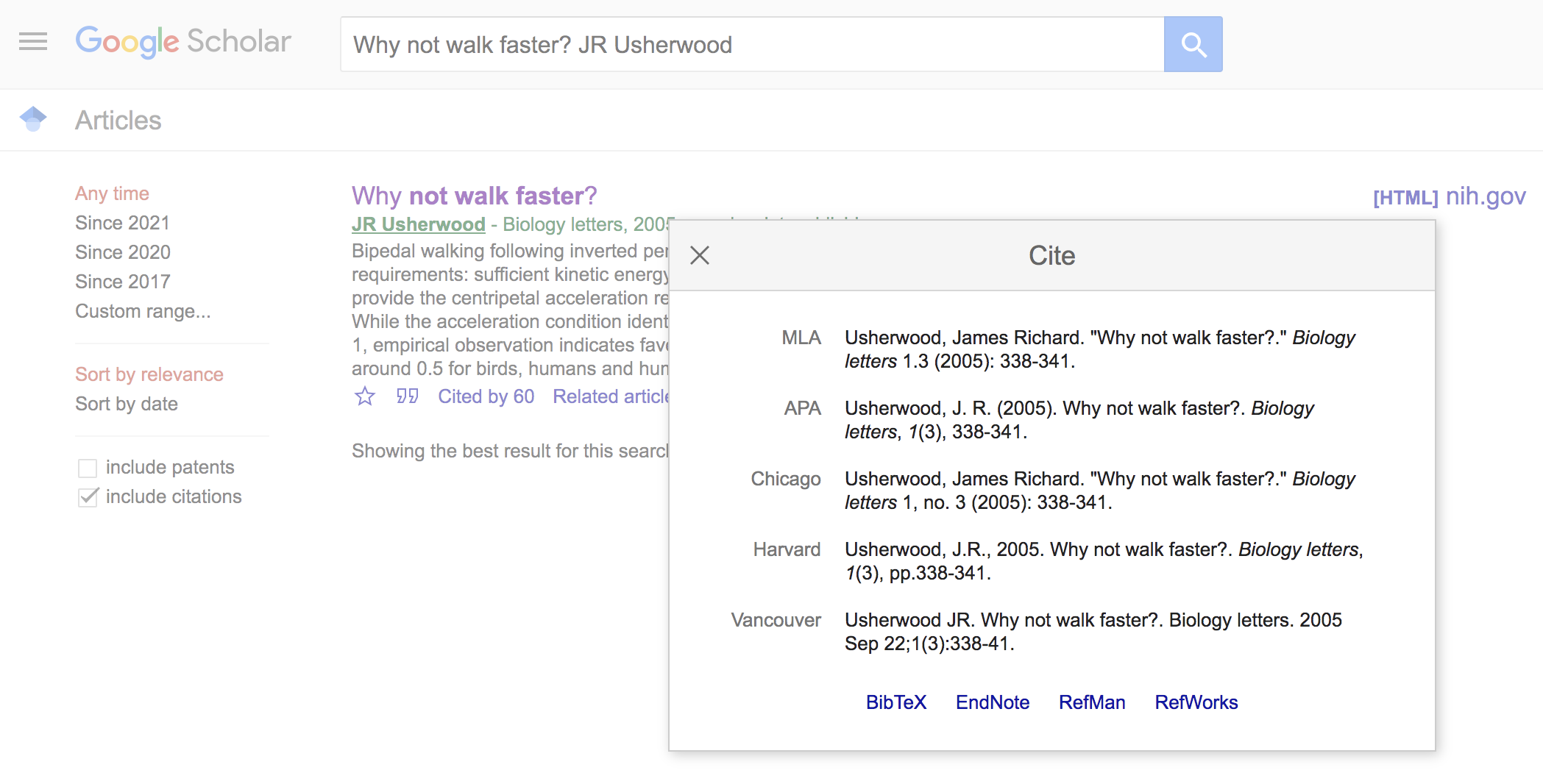
Now click on the “BibTex” link at the bottom of the window. This will lead you to a text file of the BibTex format. Simply copy and paste this to your .bib file. Voila.
What to address in the report
- How did class-wide \(v_{max}\) (the theoretical velocity) compare to actual class-wide pendulum-type walking speed? Were any patterns related to this revealed by calculating the Froude number?
- How does actual pendulum walking speed vary with human size and sex?
- How does pelvic tilt change with increased normal walking speeds. What is the biomechanical basis for this?
- Does pelvic tilt at comfortable normal walking speeds vary with human size (i.e., mass and height) and sex?
NOTE: Make sure you enter your data as soon as possible here. Be sure to use all of these data from past classes in your analysis.
Due Date and Submission
Reports are due by Wednesday January 29th at 11:59 pm. You should compress your directory containing the .Rmd and data. This .zip file should be uploaded here.